A true Infection Finder
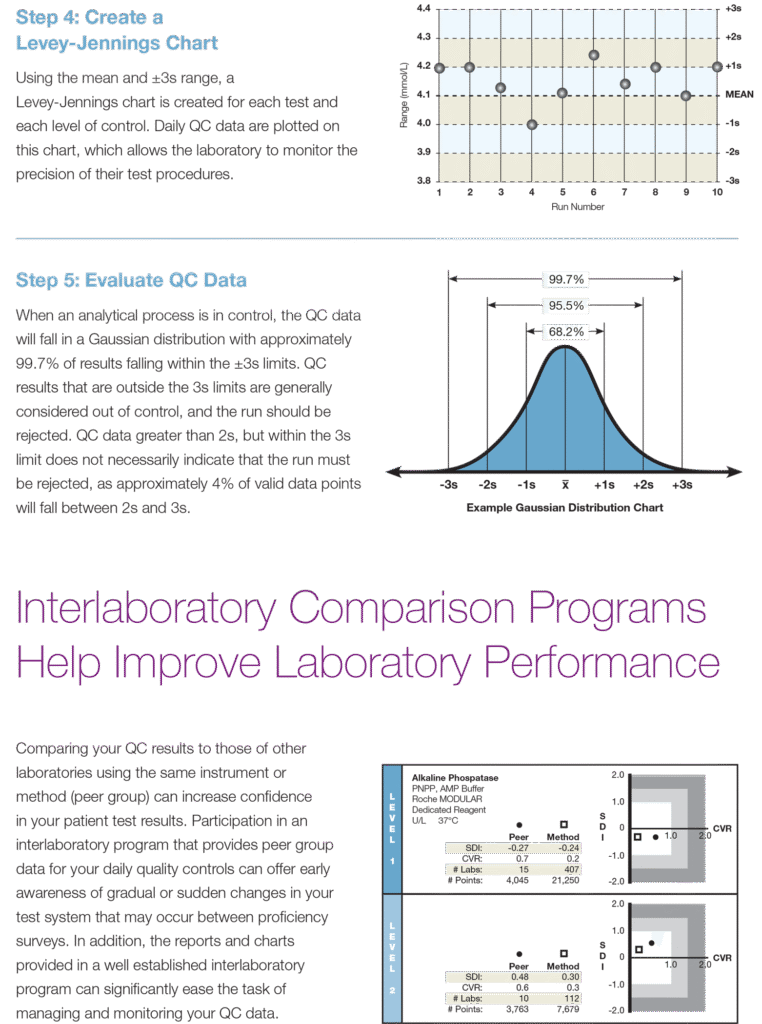
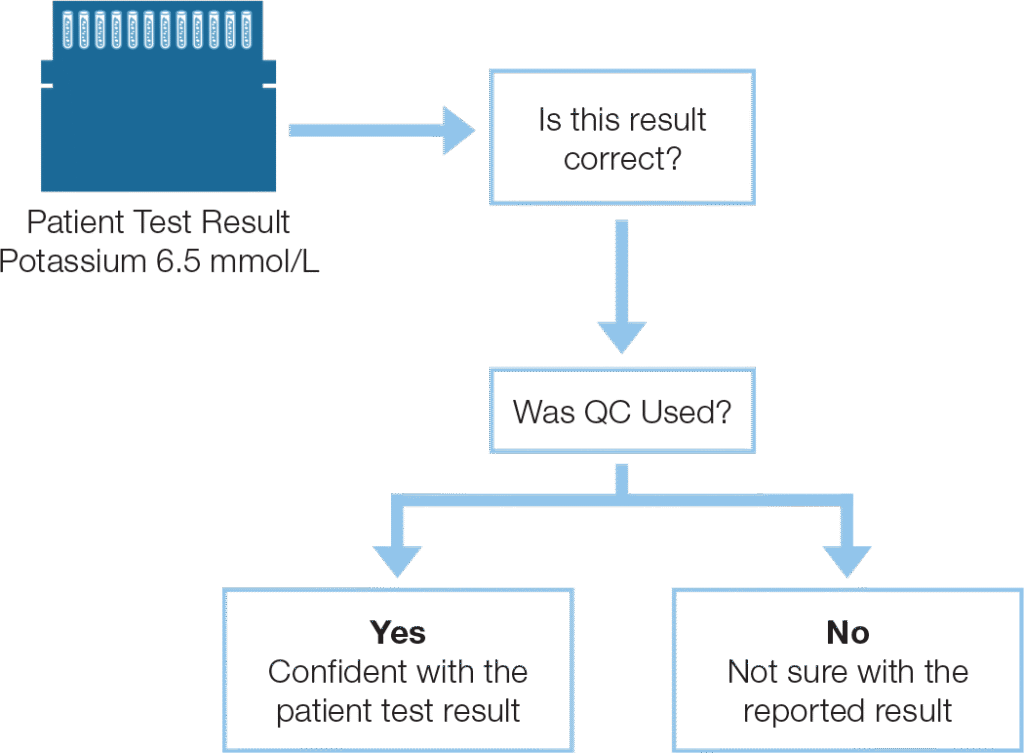
Using Quality Control
The Foundation for a Higher Standard of Patient Care Through Improved Laboratory Performance

Reliable Patient Test Results Require Daily Quality Control
Without a quality control, how do you have confidence in your patient test results?
Patient test results provided by your laboratory allow health care providers to make critical, and potentially life-saving, diagnostic and therapeutic decisions. Regular quality control testing to confirm the precision of your test systems is the foremost way to provide more confidence that your patient results are correct.
Worldwide Standards Call for Quality Control Practices
Regulations and standards across the world have specific quality control requirements or guidelines. Meeting those requirements is the responsibility of every laboratory.
ISO 15189 (International)
“The laboratory shall design internal quality control systems that verify the attainment of the intended quality of results.”
“The quality management system shall include internal quality control and participation in organized interlaboratory comparisons…”
NATA (National Association of Testing Authorities), AS 4633 (ISO 15189), Australia
“The laboratory must have a system of long term monitoring of internal quality control results to assess method performance.”
“The quality management system shall include internal quality control and participation in organized interlaboratory comparisons…”
CAP (College of American Pathologists),
Chemistry and Toxicology Accreditation Checklist, United States
“Control results must be reviewed before reporting patient/client results. It is implicit in
quality control that patient/client test results will not be reported
when controls do not yield acceptable results.”
“In general, calibrators should not be used as QC materials.”
Essential Standards for Registration of Medical Testing Laboratories in India, Quality Council of India
“Medical laboratories shall perform internal quality control. Use of third party human matrix
quality control is recommended for all analytes.”
NABL (National Accreditation Board for Testing & Calibration Laboratories)
“The laboratory shall include a minimum of one level of QC at least once a day.” (or more if >25 patient samples are analyzed per day).
CLIA (Clinical Laboratory Improvement Amendments), United States
“The laboratory must … establish or verify the criteria for acceptability of all control materials.”
“Perform control procedures . . . at least once each day patient specimens are assayed . . .”
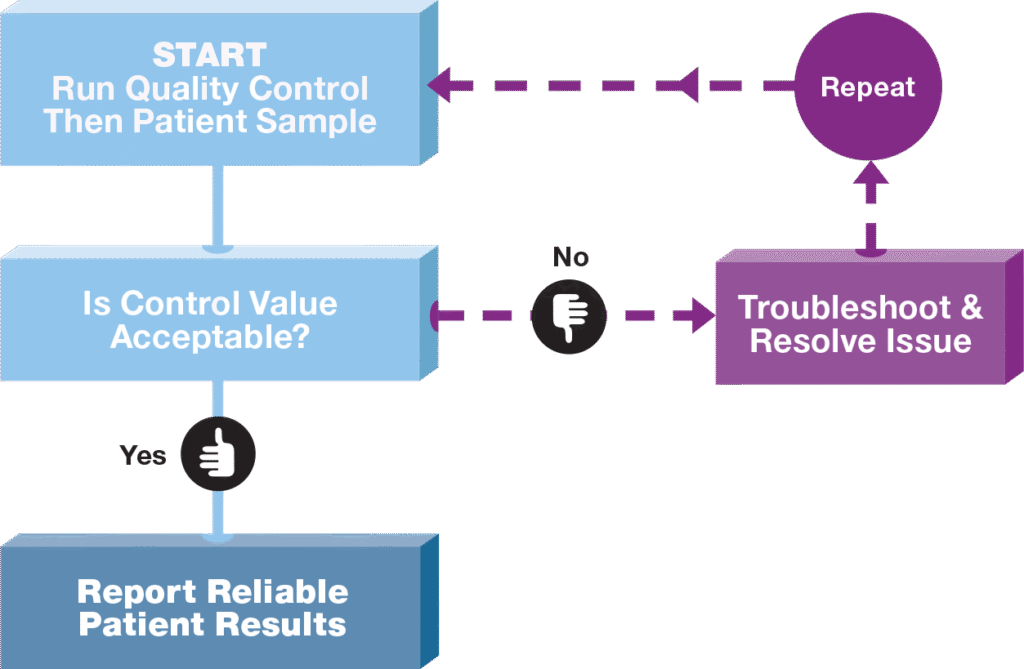
The Starting Point to a Successful Quality Control System
Implementing the use of quality controls on a daily basis in your laboratory is a sound investment in better patient care. A simple, yet effective QC system can help insure that your patient test results are reliable.
An External Proficiency Program is Not Enough
Routine use of daily quality controls is the foundation for a higher standard of patient care through improved laboratory performance. External proficiency programs, or external quality assessment (EQA) schemes, also play an essential role in assuring laboratory quality by supporting daily QC, but are not enough when used alone. EQA schemes typically involve testing unknown samples on a monthly or bi-weekly basis, and as such can only provide information about the testing performed on that particular assay run, on that particular day.
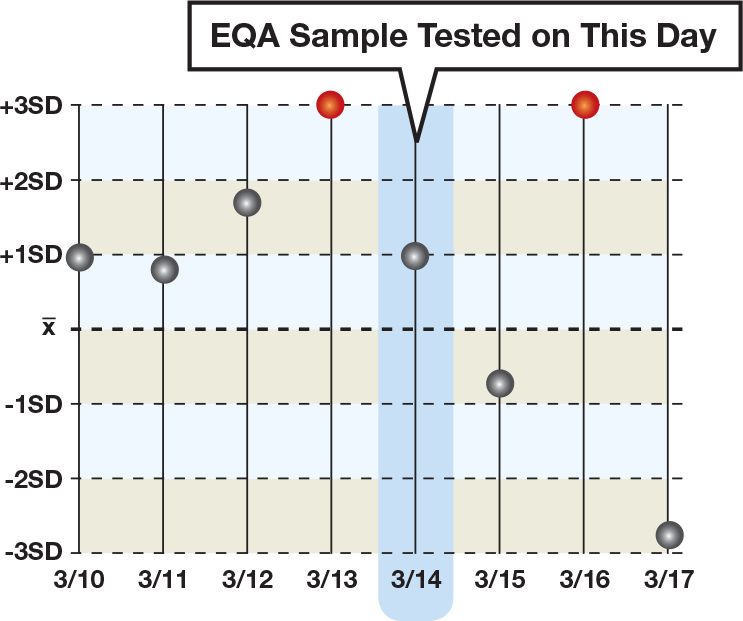
What about the many tests that are run in between EQA samples? How can the laboratory be sure of these patient test results?
An EQA program provides valuable information that is relevant to a specific moment in time. When used in conjunction with daily controls, the laboratory has a more complete picture of assay performance.